I don’t even know where I made mistakes… I end up with the same answer even though i did many ways to get answer 


 help me pls
help me pls 




Thermochemistry Question
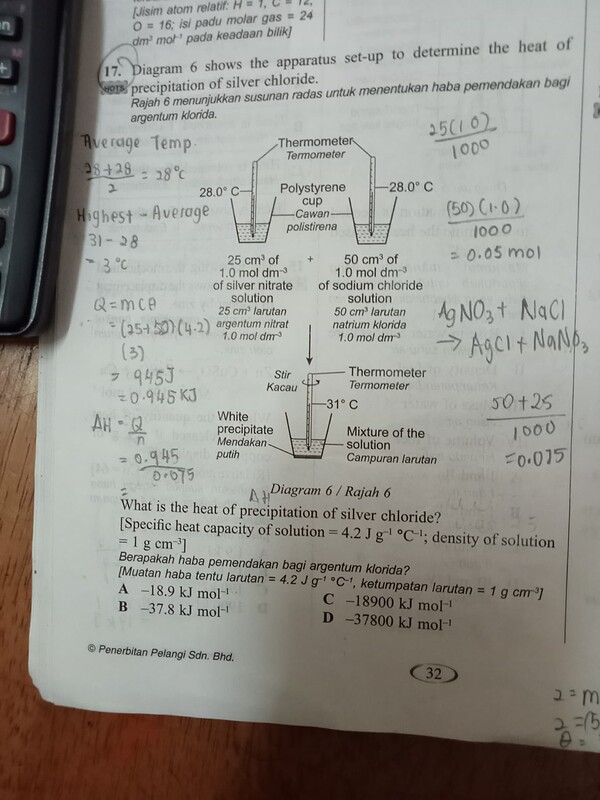
- Diagram 6 shows the apparatus set-up to determine the heat Or ofs precipitation of silver chloride.
Rajah 6 menuniukkan susunan radas untuk menentukan haba pemendakan bagi argentum kionida.
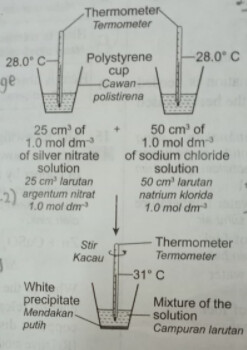
What is the heat of precipitation of silver chloride? [Specific heat capacity of solution =4.2 \mathrm{~J} \mathrm{~g}^{-1}{ }^{\circ} \mathrm{C}^{-1}; density of solution \left.=1 \mathrm{~g} \mathrm{~cm}^{-3}\right]
Berapakah haba pemendakan bagi argentum klorida?
[Muatan haba tentu larutan =4.2 \mathrm{Jg}^{\prime}{ }^{\circ} \mathrm{C} \mathrm{C} :, ketumpatan larutan =1 \mathrm{~g} \mathrm{~cm}^{-1} )A -18.9 \mathrm{~kJ} \mathrm{~mol}^{-1}
C -18900 \mathrm{~kJ} \mathrm{~mol}^{-1}
B -37.8 \mathrm{~kJ} \mathrm{~mol}^{-1}
D -37800 \mathrm{~kJ} \mathrm{~mol}
Thermochemistry Solution
Step 1 : Write the chemical equation.
AgNO3 + NaCl ----> AgCl + NaNO3
Step 2 : Determine number of mole of the formed precipitate [AgCl]
Formula :
n=MV/1000
Number of moles of Ag+ = Number of moles of AgNO3
Number of mole of Ag+ = ( 1.0 ) ( 25 ) /1000 = 0.025 mol
Number of moles of Cl- = Number of moles of NaCl
Number of mole of Cl- = (1.0) (50) / 1000 = 0.05 mol
We must choose the smallest number of moles which is 0.025 mol
Step 3 : Determine the change of heat
Formula :
Q= mc0
Temperature change: 31 -28 = 3°C
Specific Heat capacity = 4.2
Total mass of solution = 25.0cm3 + 50.0 cm3 = 75cm3 ( 1g/cm3 )= 75g
Q=(75)(4.2)(3)
Q=945 J
Q=0.945kJ
Step 4 : Calculate the heat of precipitation
Formula :
∆H = -Q/n kJ mol-1
∆H ( Heat of precipitation) = - (0.945)/ (0.025) = -37.8 kJ mol-1
ANSWER : B
My god … U are such a life saver …


I’m like wondering why I end up with different answer… Now only I understand that I had choose the smallest value… I had add (tambah) kedua - dua value ni!
 Now I understand ready … Thank u so much … Beribu berterima kasih kepada kawan yang tolong saya ni
Now I understand ready … Thank u so much … Beribu berterima kasih kepada kawan yang tolong saya ni 





You are welcome dear!