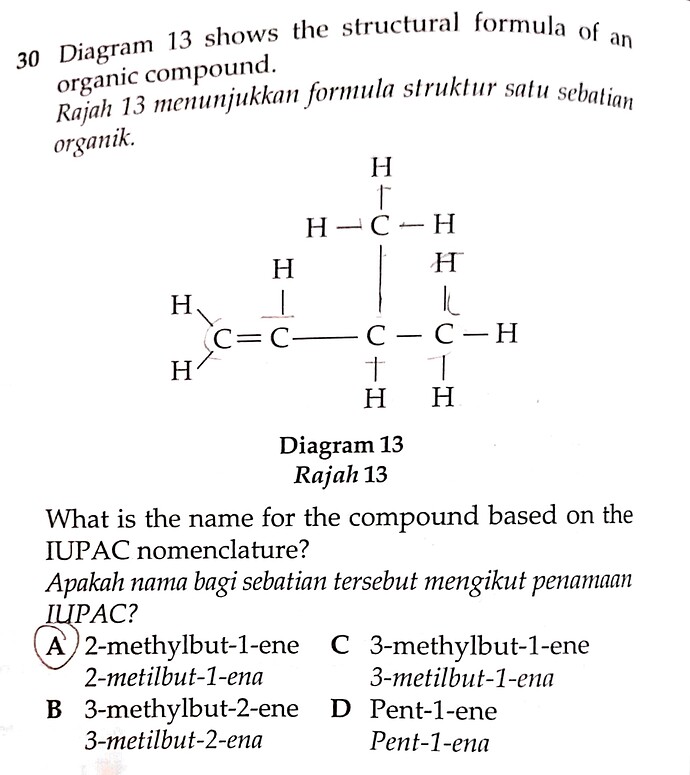
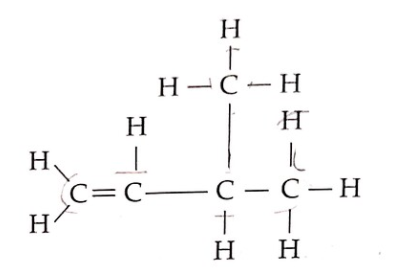
30 Diagram 13 shows the structural formula of an organic compound.
Rajah 13 menunjukkan formula struktur satu sebatian organik.
Diagram 13 Rajah 13
What is the name for the compound based on the IUPAC nomenclature?
Apakah nama bagi sebatian tersebut mengikut penamaan IUPAC?
A. 2-methylbut-1-ene / 2-metilbut-1-ena
B. 3-methylbut-2-ene / 3-metilbut-2-ena
C. 3-methylbut-1-ene / 3-metilbut-1-ena
D. Pent-1-ene / Pent-1-ena
Hi Farhaini,
Referring again to: [SPM Chemistry Hydrocarbon Q40 Solution] Carbon Carbon compound - #2 by Alex
We need to prioritize naming of double bond (lower carbon numbering) in this case, since it is a functional group.
- The longest carbon chain has four carbons, with a double bond, so it belongs to the ‘butene’ (C4) family.
- Identify the presence of any double bond on the lowest carbon number. For this question, Since the double bond is at the first carbon, it should be renamed but-1-ene.
- Following the above logic, the methyl group is on the third carbon, so it should be named as 3-methyl.
Combining all the information above, the final IUPAC name would be C. 3-methylbut-1-ene.
In case you aren’t sure, IUPAC naming convention is as follows from Wiki:
- Identification of the parent hydrocarbon chain. This chain must obey the following rules, in order of precedence: (refer to Wiki)
- Identification of the parent functional group, if any, with the highest order of precedence. Example of functional group would be the ‘alkene’ group or double bond.
- Identification of the side-chains. Side chains are the carbon chains that are not in the parent chain, but are branched off from it.
Ohh okay now I understand. Thank you very much !