Hi, may I know what is the correct answer for this question? The book gives the answer D, but this question is asking about elements in Period 3. Isn’t the answer should be C, Group 18, as Chlorine can form several oxides? Thank you! (P/s: I posted the remaining question at ‘reply’)
Question
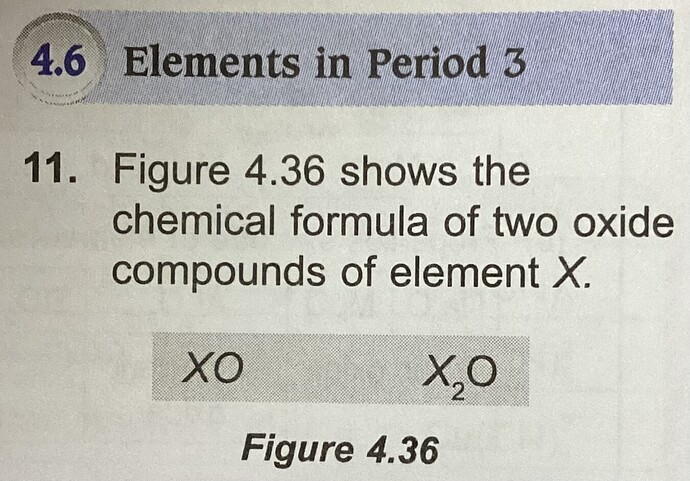
Figure 4.36 shows the chemical formula of the two oxide compounds of element X.
XO
X_2O
Element X is located in which group of the Periodic Table of element?
A) Group 1
B) Group 17
C) Group 18
D) Transition elements
Answer
Hi Jane,
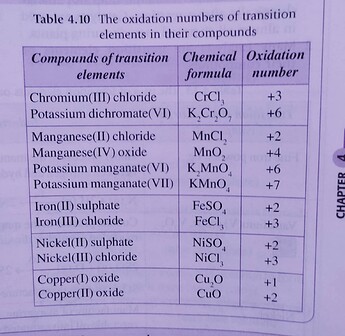
From XO and X_2O, we can deduce that X has variable oxidation numbers.
This is because the oxidation number of O is (-2).
For XO, the overall oxidation number in the oxide compound is 0, so it would mean that the oxidation number of X in XO is +2.
For X_2O, the overall oxidation number in the oxide compound is 0, so it would mean that the oxidation number of X in X_2O is +1, as 2(+1) + (-2) = 0.
We need an element that has oxidation states +1 and +2.
Our answer is D because transition elements have variable oxidation states.
Here you can see that copper is a good fit for element X.
(Fun fact: copper(I) oxide is red, but copper(II) oxide is blue  )
)
(A) cannot be the answer because group 1 elements only have oxidation states +1 (e.g. Li^+, K^+, Na^+) and oxidation states 0 (in atoms, e.g. Li, Na, K)
(B) cannot be the answer, group 17 elements like chlorine have common oxidation numbers of +1,+3,+5,+7 and -1. (Not sure if you learned this)
If you search this on Wikipedia, you do see ClO and Cl_2O, but if you click on ClO it is actually a radical compound, so to be more precise it should be ClO• not ClO, while your textbook only shows Cl_2O_7 if I’m not mistaken 
Chlorine oxide - Wikipedia
Chlorine monoxide - Wikipedia
(C) is out too because Group 18 elements are noble gases with oxidation states of 0.
Maybe the question was incorrectly categorized into that subtopic 
Thank you so much for the explanation! This book does have many mistakes😆