14.B
dissolving any ammonium salts in water is endothermic reaction
Question
14 Which of the following is an example of endothermic reaction?
Antara yang berikut, yang manakah contoh bagi tindak balas endotermik?A Solid sodium hydroxide dissolved in distilled water
Pepejal natrium hidroksida dilarutkan dalam air suling
B Solid ammonium nitrate dissolved in distilled water
Pepejal ammonium nitrat dilarutkan dalam air suling
C Dilute hydrochloric acid added to silver nitrate solution
Asid hidroklorik cair ditambahkan kepada larutan argentum nitrat
D Dilute hydrochloric acid added to potassium hydroxide solution
Asid hidroklorik cair ditambahkan kepada larutan kalium hidroksida
Answer
Hi Vincent,
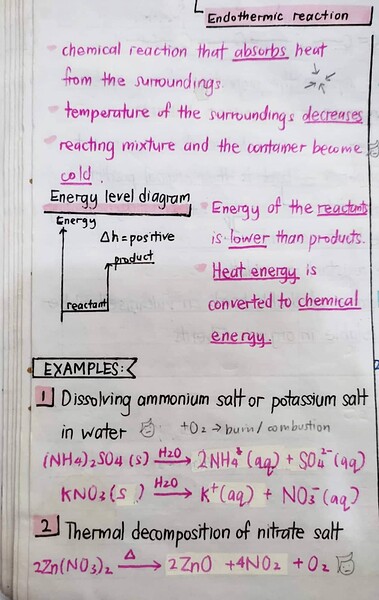
An endothermic reaction means a chemical reaction that absorbs heat from the surroundings.
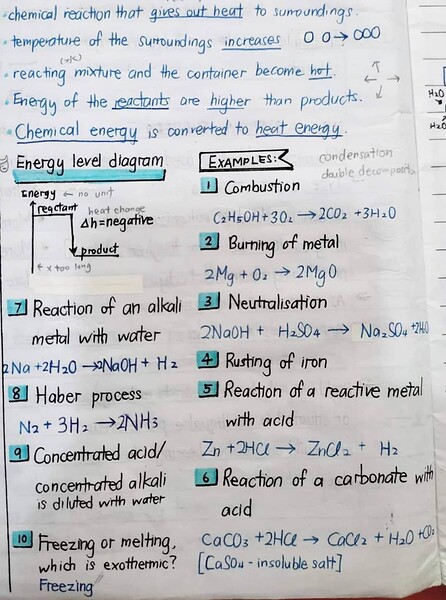
An exothermic reaction means a chemical reaction that releases heat to the surroundings.
Usually, most of the chemical reactions are exothermic. Here are some notes explaining exothermic reaction in detail and its examples:
(ignore the doodles haha I’m not sure why I drew them)
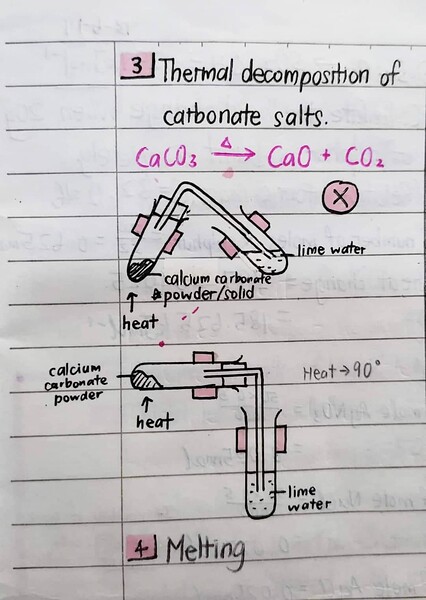
And for endothermic reaction:
We should memorize them (especially the endothermic ones) for examination purposes. So based on the notes, our answer is B, because B is an ammonium salt dissolved in water.
A is something like dilution, because we are adding water to the solid NaOH.
C falls under a precipitation reaction, where the precipitate formed is silver chloride:
HCl (aq) + AgNO_3 (aq) \rightarrow AgCl (s) + HNO_3 (aq)
(Heat of precipitation is under Form 5 Thermochemistry, this particular reaction is exothermic. If you have done it in your school laboratory, the temperature of the reaction mixture would increase when you start stirring the two solutions.)
D is a neutralization reaction, which falls under the exothermic category.
Hope this helps!