Question
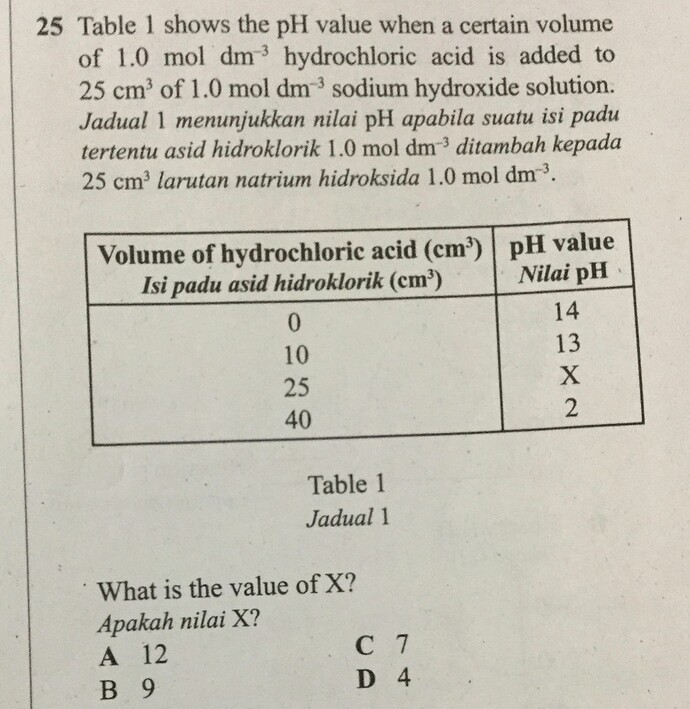
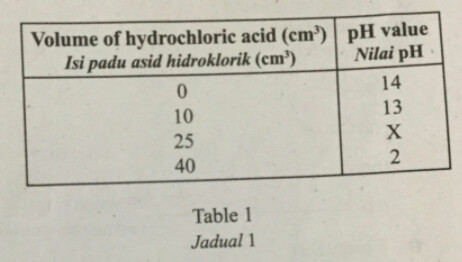
25 Table 1 shows the pH value when a certain volume of 1.0 \mathrm{~mol} \mathrm{dm}^{-3} hydrochloric acid is added to 25 \mathrm{~cm}^{3} of 1.0 \mathrm{~mol} \mathrm{dm}^{-3} sodium hydroxide solution.
Jadual 1 menunjukkan nilai pH apabila suatu isi padu tertentu asid hidroklorik 1.0 \mathrm{~mol} \mathrm{dm}^{-3} ditambah kepada 25 \mathrm{~cm}^{3} larutan natrium hidroksida 1.0 \mathrm{~mol} \mathrm{dm}^{-3}.
What is the value of X?
Apakah nilai X?
A 12
B 9
C 7
D 4
Answer
7 is neutral right…if same number of mole of acid and same number of mole of alkali
is added…it will become neutral so its 7
3 Likes