SPM 2016 Question 44
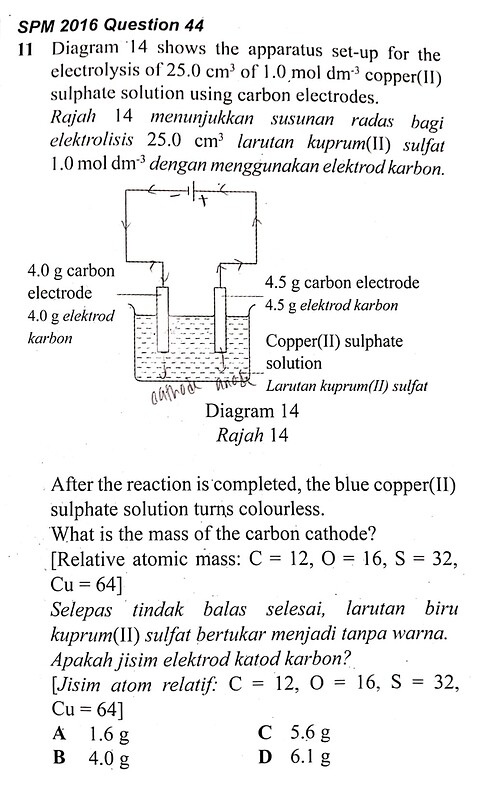
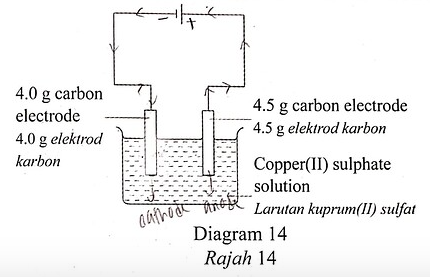
11 Diagram 14 shows the apparatus set-up for the electrolysis of 25.0 \mathrm{~cm}^{3} of 1.0 \mathrm{~mol} \mathrm{dm}^{-3} copper(II) sulphate solution using carbon electrodes.
Rajah 14 memunjukkan susunan radas bagi elektrolisis 25.0 \mathrm{~cm}^{3} larutan kuprum(II) sulfat 1.0 mol \mathrm{dm}^{-3} dengan menggunakan elektrod karbon.
After the reaction is completed, the blue copper(II) sulphate solution turns colourless. What is the mass of the carbon cathode?
[Relative atomic mass: \mathrm{C}=12, \mathrm{O}=16, \mathrm{~S}=32 \mathrm{Cu}=64]
Selepas tindak balas selesai, larutan biru kuprum(II) sulfat bertukar menjadi tanpa warna. Apakah jisim elektrod katod karbon?
[Jisim atom relatif: \mathrm{C}=12, \mathrm{O}=16, \mathrm{~S}=32 \mathrm{Cu}=64]
A 1.6 \mathrm{~g}
C 5.6 \mathrm{~g}
B 4.0 \mathrm{~g}
D 6.1 \mathrm{~g}
First, we note that the 4.0 g carbon electrode is the cathode, whereby Cu2+ ions will undergo discharge by accepted two electrons to form copper atoms and deposit copper on the electrode.
Cu2+ +2e- → Cu
Assuming reaction is fully completed, we find the number of moles of Cu deposited, based on 25 cm3 of 1.0 mol/dm3 Cu2+ solution.
Number of moles of Cu = 25 cm3 x 1.0 mol/dm3 = 0.025 dm3 x 1 mol/dm3 = 0.025 mol
Mass of Cu deposited = 0.025 mol x 64 g/mol = 1.6 g.
Therefore, the new mass of the carbon electrode is:
New mass = 4.0 g + 1.6 g = 5.6 g
Answer is C. 5.6 g
Thank you very much!