can anyone explain why is the answer 15.12dm3 plss tysm
Question
- Leaded petrol contains tetraethyllead, (C_2H_5)_4Pb. Upon a complete combustion in excess oxygen, it is converted to lead (II) oxide, carbon dioxide gas and water. Calculate the maximum volume of oxygen gas at STP needed to completely react with 16.15g tetraethyllead.
[Relative atomic mass: H=1, C=12, O=16, Pb=207; Molar volume of gas= 22.4dm^3 at STP]
Answer
Hi Isac,
When it comes to solving these type of questions, the first thing you should do is to construct a balanced chemical equation:
From the equation, we know that 1mol of tetraethyllead reacts with \frac{27}{2} mol of oxygen gas. But before knowing the moles of oxygen used, we have to find the moles of tetraethyllead used.
Since the question gave mass of tetraethyllead used and relative atomic mass of the elements, we can then find the moles of tetraethyllead used from the given information.
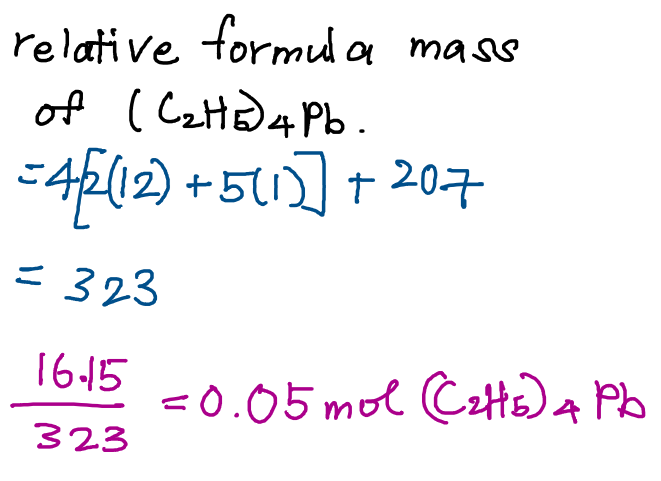
From here, we can then find moles of oxygen gas used as we have already found the mole ratio from the chemical equation.
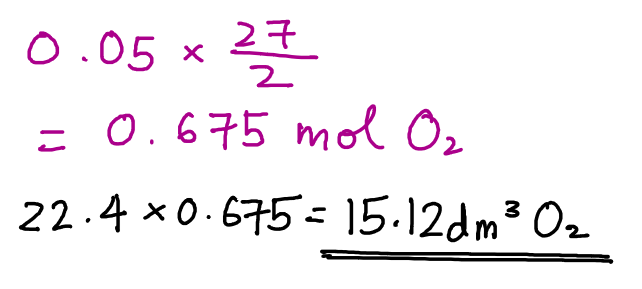
Hope this helps! Feel free to clarify if you have any questions 
Thanks a lot,Emily. I didnt think fraction can be used to balanced, got stuck at that part. Well really thanks a lot!!
Actually you can also multiply the whole equation by 2 if you’re not comfortable with fractions, then you’d get 2moles of tetraethyllead reacting with 27 moles of oxygen, the mole ratio still remains the same 
