Question
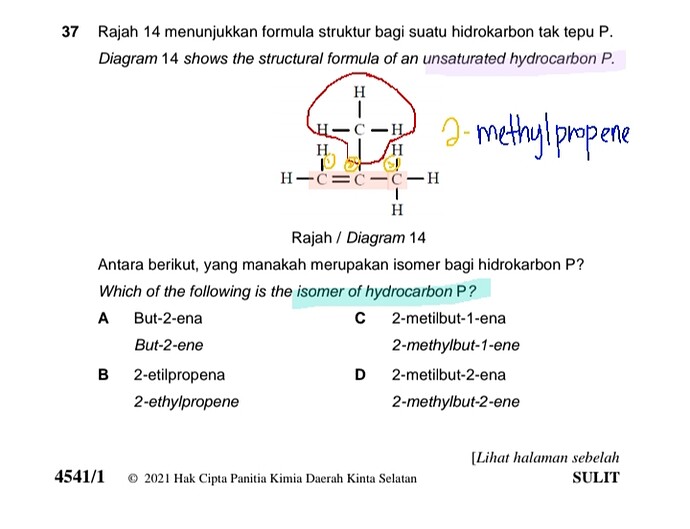
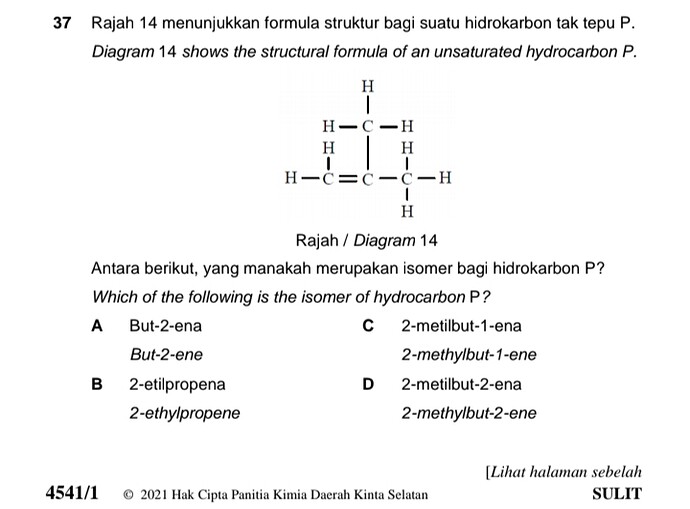
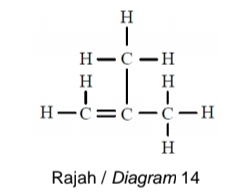
Rajah 14 menunjukkan formula struktur bagi suatu hidrokarbon tak tepu P.
Diagram 14 shows the structural formula of an unsaturated hydrocarbon P.
Antara berikut, yang manakah merupakan isomer bagi hidrokarbon P?
Which of the following is the isomer of hydrocarbon P?
A But-2-ena / but-2-ene
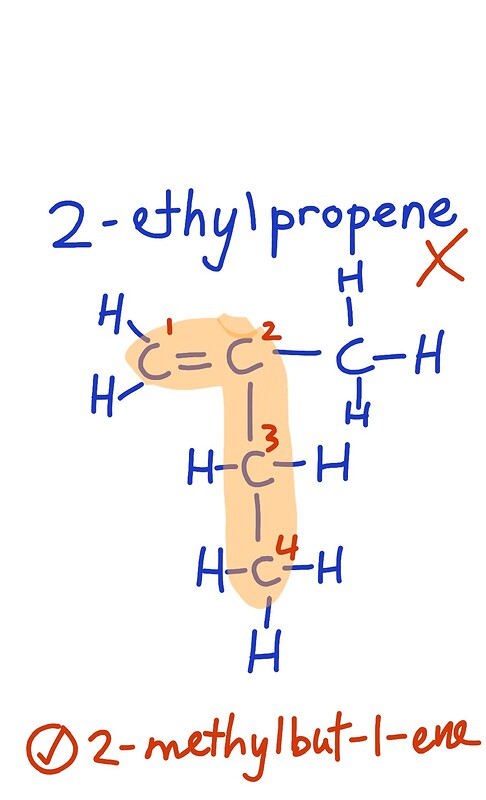
B 2-etilpropena / 2-ethylpropene
C 2-metilbut-1-ena / 2-methylbut-2-ene
D 2-metilbut-2-ena / 2-methylbut-2-ene
Answer
Hi Farhaini,
Isomers are chemical compounds with the same molecular formula but different structural formula.
2-methylpropene has a molecular formula of C_4H_8. So we have to check which option also has the molecular formula C_4H_8.
A) but-2-ene has the molecular formula C_4H_8
B) 2-ethylpropene has the molecular formula C_5H_{10} (but actually the naming is already wrong because we always take the longest carbon chain)
so it is actually 2-methylbut-1-ene.
C) and D) 2-methylbut-1-ene and 2-methylbut-2-ene each has a molecular formula of C_5H_{10}
So our answer is A.
The fastest way to see is that a methyl group has 1 carbon, ethyl group 2 carbons, propyl group 3 carbons and so on.
Then A would have 4 carbon atoms, B would have 2+3=5 carbon atoms, C
and D would have 1+4=5 carbon atoms.
Hope this helps! 
Thank you very much !