Hi Emily thanks soo much for replying. Can I ask about the theory of electronegativity and electropositivy going down group 17 halogens. OK so if it was Mr sai muns notes that he was selling promo for best students science package. His student wrote that going down the group 17 the atomic masses increased so therefore the attractions between them increase, but if it was puan farhana PTTI the atomic mass increase which means the attraction of valence electron between nucleus positive charge and negative charge becomes much weaker so the electropositivy is much greater going down the group. So which one would best in describe halogens.
Thanks maryah
Answer
Hi Maryah,
For the two cases, they are talking about different properties.
Concept 1: intermolecular forces of attraction
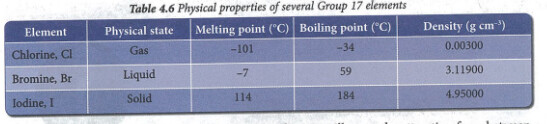
The first one (Mr Sai Mun’s) is referring to van der Waal’s forces of attraction or intermolecular forces of attraction. Intermolecular means “between molecules”.
Personally, I wouldn’t say atomic mass for Group 17 but rather molecular size because Group 17 elements exist as molecules. (I think your textbook uses molecular size too - Form 4 Chemistry Textbook page 93)
Molecular size increases down Group 17, so intermolecular forces of attraction increase down Group 17. (Not sure if the new syllabus touches on why vdW forces increase with increasing molecular size, this has to do with the size of the electron cloud and the temporary dipoles induced - more on that when you study Pre-U)
Example:
Iodine molecules, I_2 are bigger than bromine molecules, Br_2.
So the van der Waal’s forces of attraction between iodine molecules is stronger than bromine molecules.
More heat energy is required to overcome the intermolecular forces of attraction between iodine molecules than that of bromine molecules.
Iodine has a higher melting and boiling point than bromine.
This explains why iodine is a solid at RTP while bromine is a liquid at RTP.
Concept 2: Electronegativity / Electropositivity
The second one (Puan Farhana’s) has to do with electronegativity / electropositivity. Another different concept.
Electronegativity refers to the tendency of an atom to attract electrons in a molecule. The higher the electronegativity, the easier for the atom to attract electron(s) towards itself.
Electropositivity refers to the tendency of an atom to donate electrons to form a positively charged ion.
Usually, we use electropositivity for metals and electronegativity for non-metals.
So for group 17, electronegativity would be the more appropriate term.
However, we don’t use molecular size but atomic size when talking about electronegativity / electropositivity because it is the atom itself that accepts / donates electrons, not the molecule.
A sample explanation would be:
Atomic size/radius increases down group 17.
The distance between nucleus and valence electrons increases.
The attraction between the positively charged nucleus and the negatively charged valence electrons becomes weaker because of it.
Electronegativity decreases down group 17. (The attraction between the nucleus and existing valence electrons is already weak, so it is definitely harder for the atom to accept more electrons)
Reactivity decreases down group 17.
This explains why chlorine is more reactive than iodine, as it is harder for an atom like iodine atom to accept electrons compared to an atom like chlorine atom.
Generally, if the question asks for the reason why a group 17 element has a higher melting point / boiling point than the other, we explain it in terms of van der Waal’s forces / intermolecular forces.
If the question asks for the reason why a G17 element is more reactive than the other, we explain it in terms of electronegativity.
Hope this clears your doubts! It is always best to check out the mark scheme so you don’t miss out any important keywords. 