Question
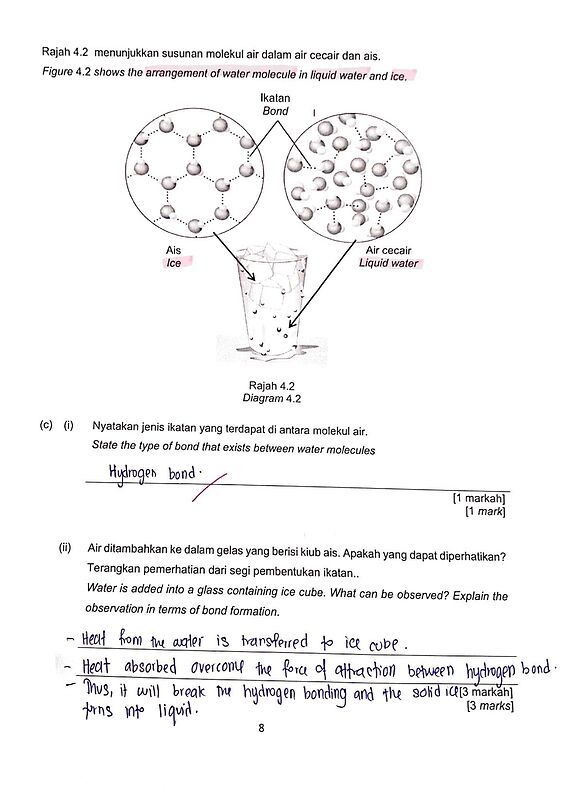
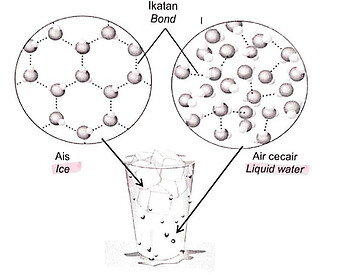
Rajah 4.2 menunjukkan susuan molekul air dalam air cecair dan ais.
Figure 4.2 shows the arrangement of water molecule in liquid water and ice.(ii) Air ditambahkan ke dalam gelas yang berisi kiub ais. Apakah yang dapat diperhatikan? Terangkan pemerhatian dari segi pembentukan ikatan.
Water is added into a glass containing ice cube. What can be observed? Explain the observation in terms of bond formation.
Answer
Hi Farhaini,
For this question there are two parts, where the first part is the observation and the second part is the explanation in terms of bond formation - what do you see and why?
So the first point of the answer is the observation when water is added to ice:
Floating ice.
You don’t ever see ice sink to the bottom of the glass, but always on the surface of the water, why?
Points 2 and 3 explain it.
Ice and water both have hydrogen bonds. But there are more hydrogen bonds in ice and the rigid crystal lattice in ice makes the hydrogen bonds stronger, hence “When water freezes, hydrogen bonds are more stable”. It is also because H_2O molecules have more kinetic energy in water compared to ice which makes weaker hydrogen bonds in water because the molecules move around more.
From the arrangement of molecules in ice and water in the diagram, you can see that the arrangement in ice is more spaced out, hence “Volume of ice is greater than that of water”
And because density is inversely proportional to volume for a fixed mass, the density of ice is lower than the density of water.
Hope this helps! 
Thank you very much!