Question
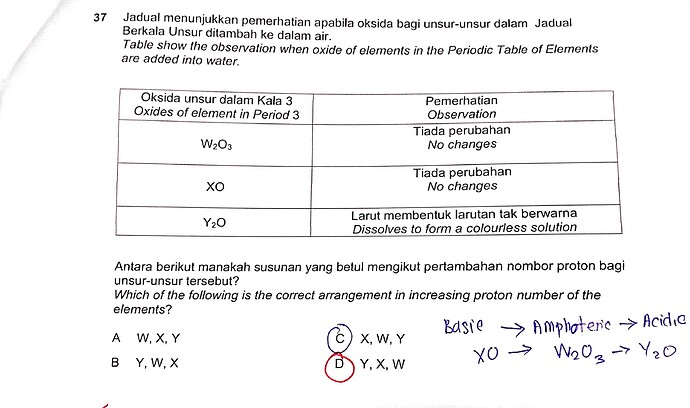
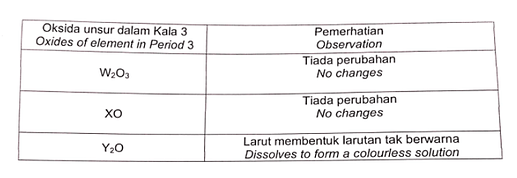
Jadual menunjukkan pemerhatian apabila oksida bagi unsur-unsur dalam Jadual Berkala Unsur ditambah ke dalam air.
Table shows the observation when oxide of elements in the Periodic Table of Elements are added into water.
Antara berikut manakah susunan yang betul mengikut pertambahan nombor proton bagi unsur-unsur tersebut?
Which of the following is the correct arrangement in increasing proton number of the elements?
A W,X,Y
B Y,W,X
C X,W,Y
D Y,X,W
Answer
Hi Farhaini,
This question is most likely talking about Period 3 oxides.
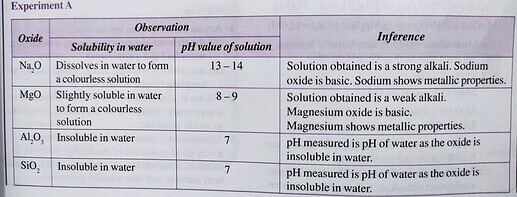
You cannot tell if an oxide is acidic or basic by adding it into water, you can only tell if an oxide is acidic or basic by adding it into an acidic solution or alkaline solution. We know that among Period 3 oxides, only 3 of the oxides of elements do not completely dissolve in water. These are MgO, Al_2O_3 and SiO_2.
From the table in the question, the oxides of W and X have no changes when they are added to water, meaning they do not react nor dissolve in water. Therefore we can conclude from the chemical formula that W is most definitely aluminium, and X is magnesium. Y is sodium because there isn’t any other Period 3 oxide with the formula Y_2O besides sodium oxide. From the Periodic Table, we know that the order in increasing proton number would be sodium, magnesium followed by aluminium. Thus the answer is Y, X,W. So this question is actually pure memorizing I would say, basically just remember the chemical formula of the Period 3 oxides and you should be fine!
Hope this helps! 
Ohhhh I seee Thank you very much! Really appreciate your help!