Hi I don’t really understand what this table means can anyone explain how to do this question thanks a lot
Question
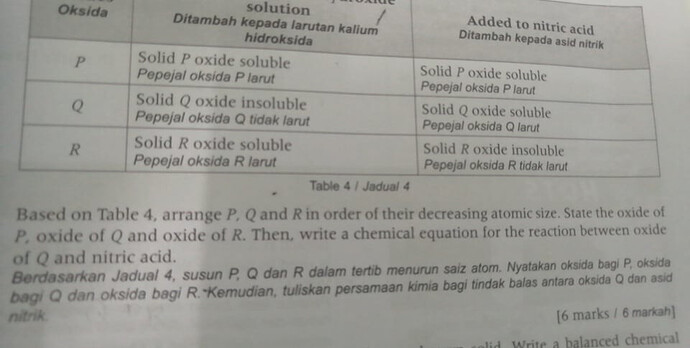
Based on Table 4, arrange P, Q and R in order of their decreasing atomic size. State the oxide of P, oxide of Q, oxide of R. Then write a chemical equation for the reaction between oxide of Q and nitric acid.
Berdasarkan Jadual 4, susun P, Q dan R dalam tertib menurun saiz atom. Nyatakan oksida bagi P, oksida bagi Q dan oksida bagi R. Kemudian, tuliskan persamaan kimia bagi tindak balas antara oksida Q dan asid nitrik.
Answer
Hi Isac,
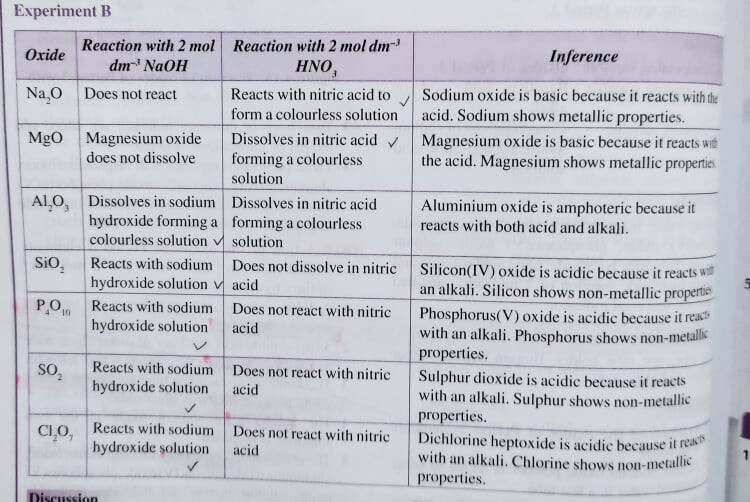
Basically this question is asking about natures of oxides.
There are 3 natures of oxides, namely:
- Basic oxide - it reacts with acids to form salt and water.
- Amphoteric oxide - it reacts with both acids and alkali to form salt and water.
- Acidic oxide - it reacts with alkali to form salt and water.
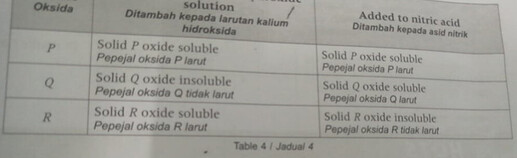
I’m not sure if there is a sentence before the table since the image is cropped, but I’m assuming they are talking about the oxides of elements in Period 3.
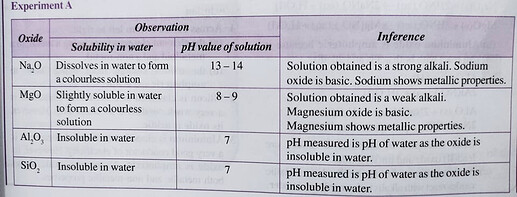
Sodium oxide and magnesium oxide are basic oxides,
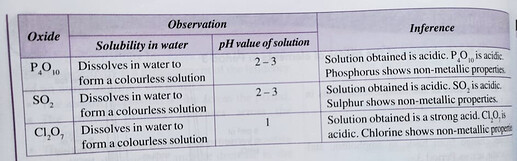
aluminium oxide is an amphoteric oxide and oxides of period 3 elements from silicon to chlorine are acidic oxides.
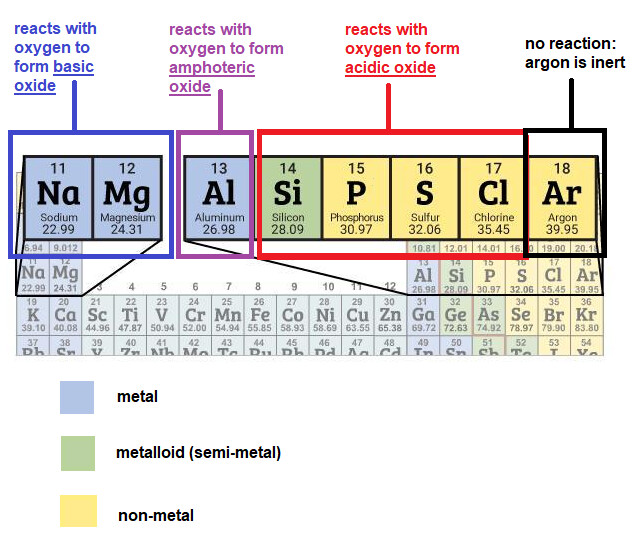
So to answer the first part of the question: arranging P, Q and R in order of decreasing atomic size.
We first have to identify which type of oxide is oxide P, Q, R.
From the table, we are able to deduce that:
oxide P is an amphoteric oxide
oxide Q is a basic oxide
oxide R is an acidic oxide
From the colourful diagram, we notice that as atomic size increases, the oxides of Period 3 elements change from basic to amphoteric to acidic,
thus order of decreasing atomic size would be the backward sequence- from acidic oxide to amphoteric oxide to basic oxide, that is: R, P, Q.
Oxide of P must be aluminium oxide, since there is only one amphoteric oxide in Period 3. But how do we know what is oxide Q and oxide R?
(If the cropped part did mention something about P, Q, R being consecutive elements in Period 3 OR oxides P, Q, R are slightly soluble / insoluble in water, then oxide Q would definitely be magnesium oxide and oxide R would definitely be silicon oxide.)
But if it did not, the best answer should still be the same, just that I couldn’t really find a good explanation for it. If you have this chemistry reference book (Success SPM- one of the best chemistry reference books for KBSM syllabus), notice that in the image below, the book specifically used dissolve instead of react for MgO, Al_2O_3, SiO_2
Not sure if the KSSM syllabus has mentioned anything in detail about this, but if it didn’t it should still be accepted if you write NaO for Q and any one of P_4O_{10}/ SO_2/ Cl_2O_7 for R
Using Q as magnesium, the chemical equation is:
MgO + 2HNO_3 -> Mg(NO_3)_2 + H_2O
Hope this helps, sorry if this is a bit long-winded haha 
Best if you could confirm it with your teacher since KSSM Chemistry syllabus differs slightly from the KBSM syllabus 
Yes the sentence before the table is talking about observation when three oxides of elements in period 3 only. thank you so much for the explanation