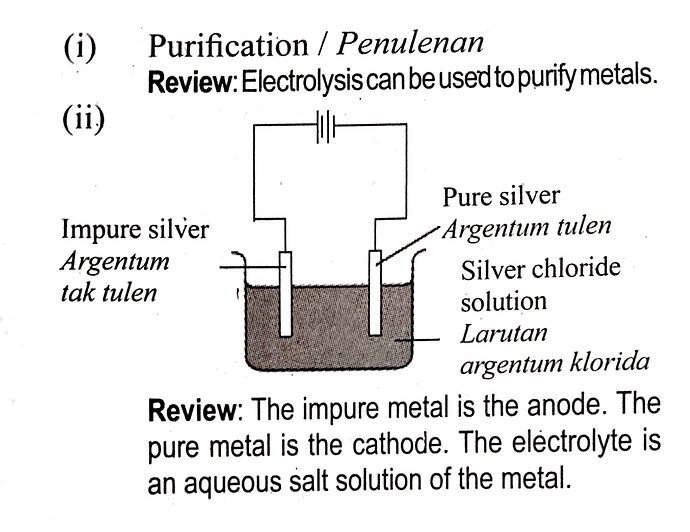
How to determine the aqueous salt solution of the metal (electrolyte)? Silver chloride is an insoluble salt so it is the aqueous solution must be an insoluble salt?
(e) A metal Z is found containing some impurities. Z is located below copper in the electrochemical series. Suatu logam Z didapati mengandungi sedikit bendasing. Z terletak di bawah kuprum dalam siri elektrokimia.
(i) State the method used to purify metal \mathrm{Z}.
Nyatakan kaedah yang digunakan untuk menulenkan logam Z itu. [1 mark ] [1 markah ]
(ii) Draw a labelled diagram for the apparatus set-up for \mathbf{3}(e)(\mathrm{i}). Lukis rajah berlabel untuk susunan radas bagi \mathbf{3}(e) (i). [3 marks ] [3 markah ]
Based on the reactivity series, only silver, mercury or gold are lower than copper.
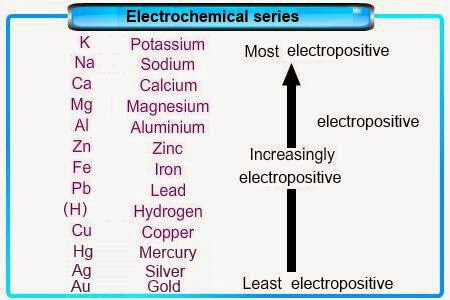
For metal purification, usually the impure metal will be used as the anode, because the metal will undergo ionization at the anode, and then deposit on the cathode as ‘pure metal’. For example:
M → M⁺ + e⁻
In this case, we therefore would want a metal solution of M⁺, which must be an aqueous solution consisting of M⁺ ions. Assuming silver as the metal of interest, only silver nitrate, AgNO3 solution would be suitable as an electrolyte.
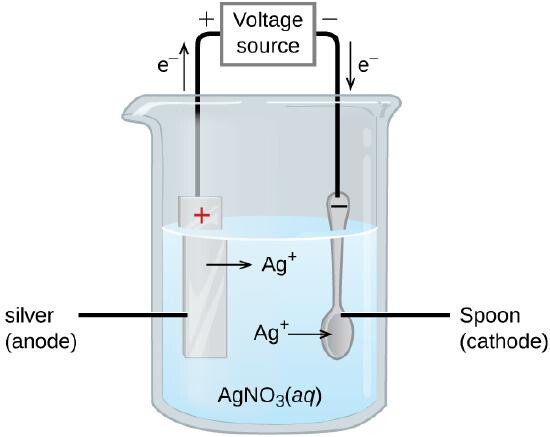
I believe the answer, silver chloride solution is wrong as AgCl is insoluble in water.
Ouhh so the electrolyte must be soluble in water.Thankyou very muchh