Chemistry Question
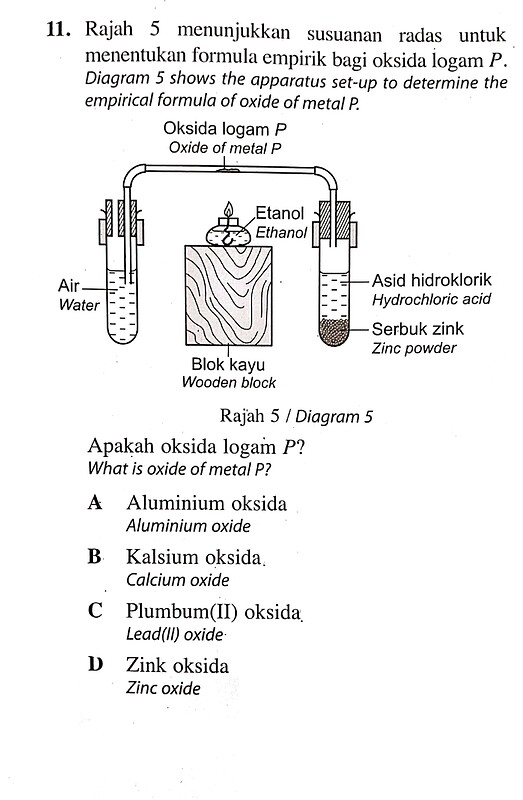
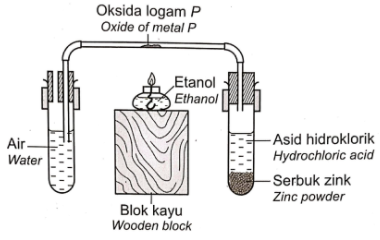
- Rajah 5 menunjukkan susuanan radas untuk menentukan formula empirik bagi oksida logam P. Diagram 5 shows the apparatus set-up to determine the empirical formula of oxide of metal \mathrm{P}.
Rajah 5 / Diagram 5
Apakah oksida logam P ? What is oxide of metal P ?
A Aluminium oksida
Aluminium oxide
B Kalsium oksida
Calcium oxide
C Plumbum(II) oksida
Lead(II) oxide.
D Zink oksida
Zinc oxide
Chemistry Solution
First, we note that the reaction between zinc powder and hydrochloric acid produces hydrogen in accordance to the following chemical reaction.
Zn + 2HCl → ZnCl₂ + H₂
Next, the hydrogen gas produced will reduce the oxide of metal P in the presence of heat (produced by the burning ethanol), producing metal P and water.
PO + H₂ → P + H₂O
Only metals with lower reactivity than hydrogen in the reactivity series can be reduced by hydrogen.
The chart below shows the position of carbon and hydrogen in the reactivity series of metal based on their ability to attract oxygen to form oxide.
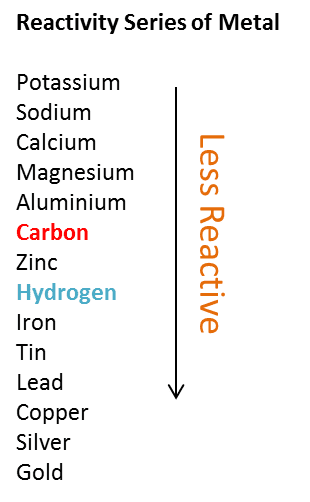
From the chart, we notice that Lead is below hydrogen, hence hydrogen can reduce lead (II) oxide, PbO to Pb.
PbO + H₂ → Pb + H₂O
Therefore, answer is C Plumbum(II) oksida.
Thank you very much !