SPM Chemistry Question 2018 \mathrm{Q} 29
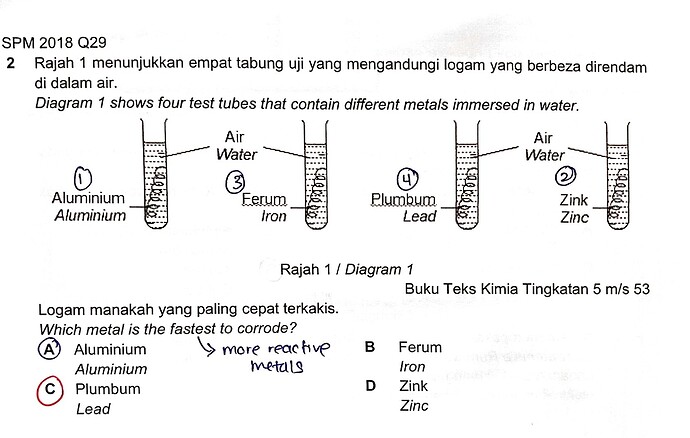
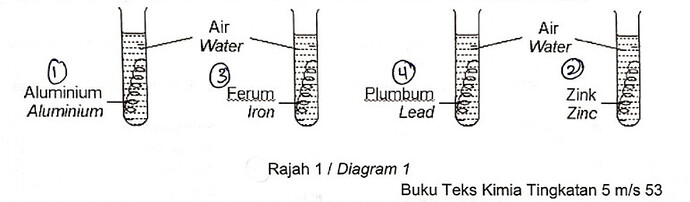
2 Rajah 1 menunjukkan empat tabung uji yang mengandungi logam yang berbeza direndam di dalam air.
Diagram 1 shows four test tubes that contain different metals immersed in water.
Logam manakah yang paling cepat terkakis.
Which metal is the fastest to corrode?
A Aluminium
Aluminium
B Ferum
Iron
C Plumbum
Lead
D Zink
Zinc
Chemistry Solution
I disagree with the answer being C. Lead does not corrode much in the presence of water. However, corrosion is not just a matter of reactivity.
In terms of which metal will ‘oxidize first’, Al > Zn > Fe > Pb which is what you have described.
- Although aluminium is the most reactive, the layer of aluminium oxide formed is corrosion-resistant thus slowing down the reaction rate.
The formation of Fe2O3 and subsequently hydrated iron (III) oxide accelerates the process of ‘corrosion’ otherwise known as rusting, so normally iron should be the metal to corrode the fastest here… Maybe there needs to be some checking with the answer?
Ouhh I see 
 …I think the same too but why zinc can’t be the answer?
…I think the same too but why zinc can’t be the answer?