Why the answer state that the concentration of Cu2+ ion will decrease…If we use copper electrode, the concentration for Cu2+ will be the same…Is it correct?
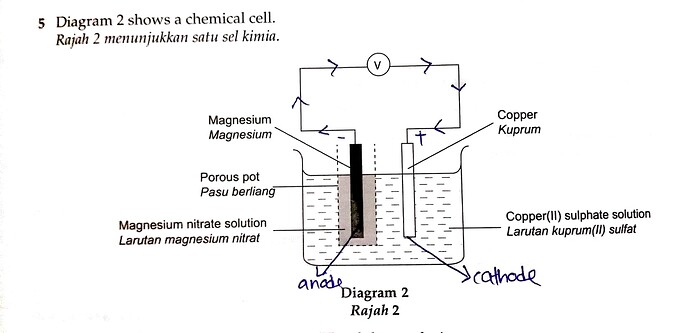
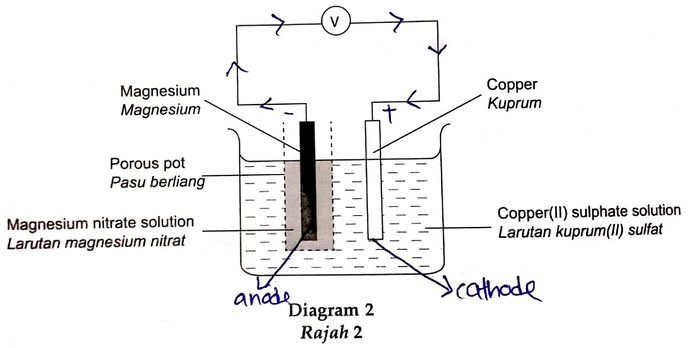
5 Diagram 2 shows a chemical cell.
Rajah 2 menunjukkan satu sel kimia.
(e) After 30 minutes, what happens to the intensity of the blue copper(II) sulphate solution? Explain your answer.
Selepas 30 minit, apakah yang terjadi kepada keamatan warna biru larutan kuprum(II) sulfat? Terangkan jawapan anda.
Explanation:
You have correctly identified that the copper electrode is acting as the cathode.
Since the electrolyte consists of Cu²⁺ ions, they will undergo discharge, combining with two electrons to form copper atoms as shown below.
Cu²⁺ + 2e⁻ → Cu
The concentration of Cu²⁺ WILL BE THE SAME only if the anode is ALSO COPPER ELECTRODE. If the anode is copper electrode, then the copper will undergo oxidation to form Cu²⁺ ions to balance the loss of Cu²⁺ ions discharged at the cathode, thus causing the intensity of blue colour to remain the same.
In this case, the anode is MAGNESIUM, therefore Mg²⁺ ions will be produced which will replace the Cu²⁺ ions discharged, causing the intensity of blue colour to decrease.
I hope this is clear 
Ohh I see …Thank you very much !