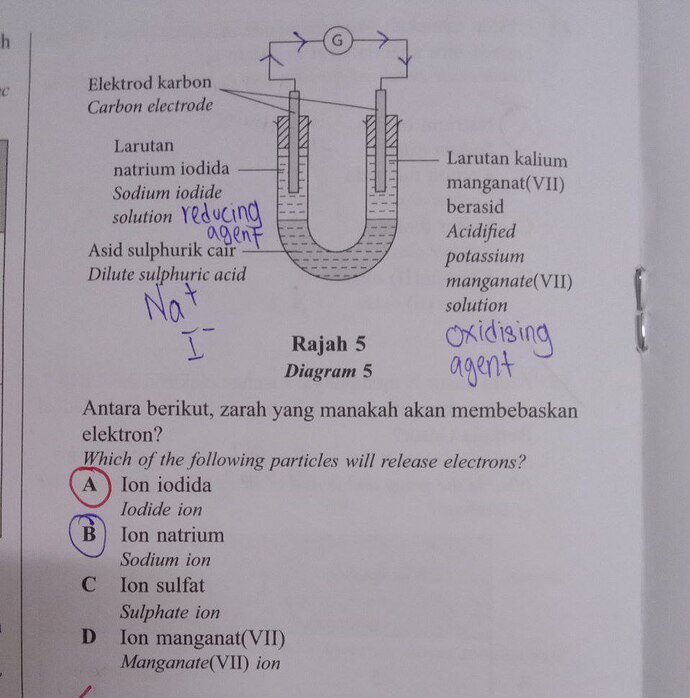
You are right! The sodium iodide (NaI) is the reducing agent and potassium manganate (VII) is the reducing agent. So oxidation happens at anode (sodium iodide loses electrons) and reduction happens at cathode (KMnO^4 gains electrons).
The half equation at anode is: 2I^{-} (aq) -> I^2 (s) + 2e^-
The half equation at cathode is: KMnO_4^- + 8H^+ + 5e^- -> Mn^{2+} + 4H_2O
Na^{+} has an extra proton. For example, sodium has 11 protons and 10 electrons and it needs to receive electrons to have a stable electron arrangement.
Na^+ (aq) + e^− → Na (s)