Question
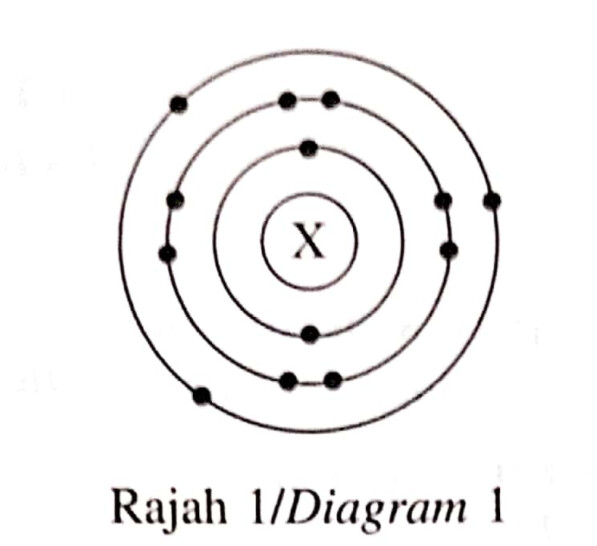
Rajah 1 menunjukkan struktur atom bagi atom X.
Diagram 1 shows the atomic structure of atom X.
Antara pernyataan berikut, yang manakah betul mengenai atom itu?
Which of the following statements about the atom are correct?
I Bilangan neutron bagi atom X ialah 14.
The number of neutrons of atom X is 14.II Susunan elektron bagi atom X ialah 2.8.4.
The electron arrangement of atom X is 2.8.4III Jisim atom relatif bagi atom X ialah 27.
The relative atomic mass of atom X is 27.IV Atom X akan menerima 5 elektron untuk mencapai susunan elektron oktet yang stabil.
Atom X will receive 5 electrons to achieve a stable octet electron arrangement.A I dan II / I and II
B I dan III / I and III
C II dan IV / II and IV
D III dan IV / III and IV
Answer
Hi Farhaini,
You know that the electron arrangement of the atom is 2.8.3, which means that there are 13 electrons in total. Since it is an atom, the proton number=number of electrons=13. To find the number of neutrons, the nucleon number should be stated in the question or refer to the periodic table if given. Aluminium has a proton number of 13, and nucleon number of 27. Since nucleon number= number of protons + number of neutrons, number of neutrons= 27-13=14.
For IV, the word “receive” 5 electrons is inaccurate because receive indicates that the compound is ionic. So for IV to be correct, it should be “Atom X will donate 3 electrons to achieve stable octet electron arrangement”
At first I was questioning why D is incorrect too, because I thought that in Al_2Cl_6 aluminium atom achieves stable octet electron arrangement by “getting” the 5 electrons from chlorine atoms. (3 covalent bonds and 1 dative bond) But for covalent compounds we usually use the word share electrons instead of receive. That’s probably why IV is incorrect.
Hope this helps! 
I just knew about this…Thank you very much!