2 Likes
Hi Laurena,
Chemistry Thermochemistry Question
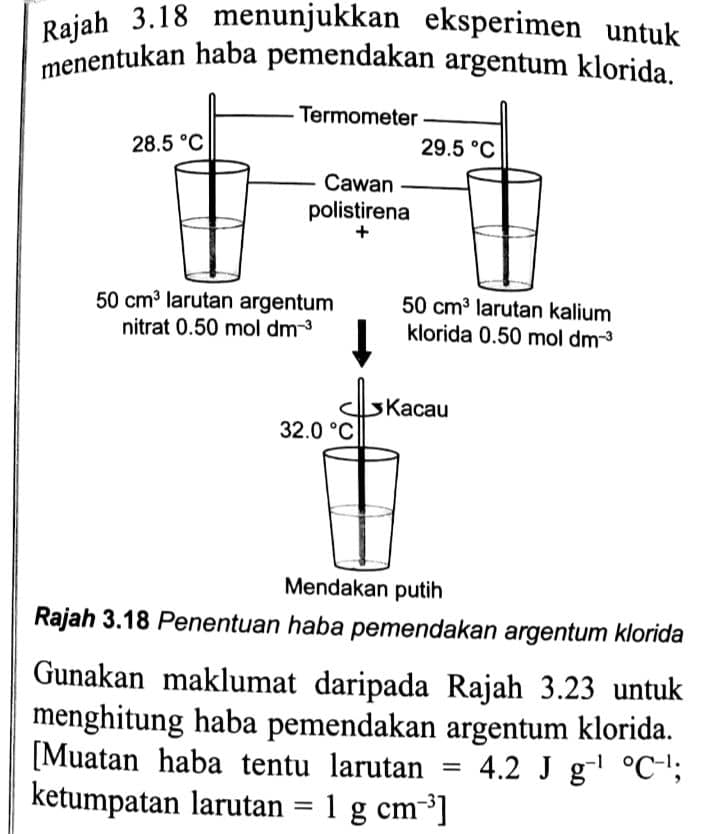
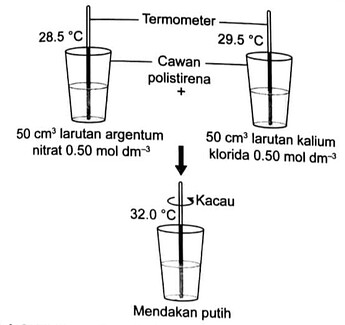
Rajah 3.18 menunjukkan eksperimen untuk menentukan haba pemendakan argentum klorida.
Rajah 3.18 Penentuan haba pemendakan argentum kloridaGunakan maklumat daripada Rajah 3.23 untuk menghitung haba pemendakan argentum klorida. [Muatan haba tentu larutan =4.2 \mathrm{~J} \mathrm{~g}^{-1}{ }^{\circ} \mathrm{C}^{-1}; ketumpatan larutan \left.=1 \mathrm{~g} \mathrm{~cm}^{-3}\right]
Chemistry Thermochemistry Solution
The question asks for heat of precipitation (haba pemendakan), which I assume must have a unit of kJ/mol as the final answer.
I will use the following steps to determine the heat of precipitation of silver chloride (haba pemendakan argentum klorida):
- Write down the chemical equation involved to identify the number of moles of AgCl formed.
- Calculate total mass of the solution, average initial temperature and temperature change
- Using Q = mcpΔT, calculate the total heat change
- Divide the total heat change, Q with the number of moles of AgCl formed to obtain heat of precipitation of silver chloride, ΔH
- AgNO3 + KCl → AgCl + KNO3
0.5 mol/dm3 x 50 cm3 = 0.5 mol/dm3 x 0.05 dm3 = 0.025 mol
Therefore, n = 0.025 moles of AgCl is produced.
- Total volume of the solution is 100 cm3. With a density of 1 g/cm3, this corresponds to m = 100 g of total mass.
The initial temperature is the average temperature of both solutions before mixing, which is:
T_initial = (28.5+29.5)/2 = 29.0 °C
The temperature change, ΔT = 32 - 29 = 3 °C
- Now, we plug in all our available values into Q = mcpΔT, using cp = 4.2 J/g°C
Q = 100(4.2)(3) = 1260 J = 1.26 kJ
- Finally, we can calculate for the heat of precipitation by dividing Q with n, assuming no heat loss to the surroundings.
ΔH = 1.26 kJ / 0.025 mol = 50.4 kJ/mol
Answer: 50.4 kJ/mol
3 Likes